All parents hope for a long and healthy life for their child. But when your child has WAGR syndrome, that hope can feel like a far-off dream. There is a way to make the dream real. Research is the key to better, healthier lives for children and adults with WAGR syndrome.
Learn more about medical research, studies involving WAGR syndrome, and how you can help turn hope into reality.
Caroline (adult with WAGR syndrome) standing on HOPE at St Jude Children's Research Hospital
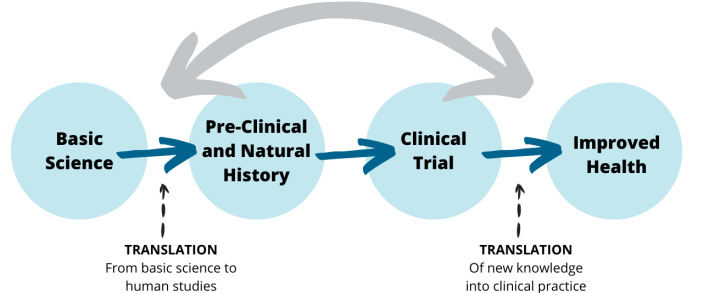
Medical research covers a wide spectrum of study types, from basic science to clinical trials. All types of studies are necessary on the path to discovering new and better treatments for the conditions associated with WAGR syndrome:
Each step in the research process leads toward the goal of improved health for people with WAGR syndrome
The reasons people participate in research differ. These reasons may include:
Informed consent is the process during which potential participants learn about the details of a study and decide whether to volunteer. The informed consent process includes:
If a participant decides to volunteer, a consent form is signed. If the participant is a child or an adult who is unable to provide consent, the permission of a parent or guardian may replace informed consent. Depending on the child's age, the child may be asked to sign or to verbally agree to participate. Adults with intellectual disability may also be asked to sign or to verbally agree to participate. If possible, children and adults with intellectual disabilities should be presented with information in a manner they can understand.
Different types of studies have varying degrees of risk. Any risks involved in a study will have been identified prior to the start of the study, and will be disclosed to the participants (see Informed Consent). All research studies that involve humans are required to undergo rigorous review by an Institutional Review Board (IRB) before the study can take place.
IRBs are made up of groups of people (scientists and non-scientists) whose job is to protect the rights and welfare of research participants and to assess risk of a particular study. Vulnerable populations, such as children or people with intellectual or developmental disabilities, are especially well protected. Prior to and during research, IRBs ensure that the study is adhering to ethical norms and that participants rights are protected.
First and foremost, people who take part in research are volunteers. At any point during a research project, a person can decide to quit the study. Study participants also have other rights.
The WAGR Syndrome Patient Registry is a collection of information about individuals diagnosed with WAGR syndrome and related disorders. The Registry is designed to help researchers better understand the conditions associated with WAGR syndrome, and to develop more effective treatments and therapies.
Partipication is free and involves completing a questionnaire that can be accessed online or requested in paper form.

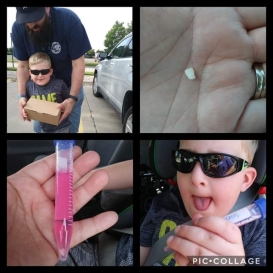
The Tooth Study is a research project to determine if neurons (brain cells) can be grown from the tooth pulp of individuals with WAGR syndrome, to better understand the nervous system defects that are common in this disorder.
To participate in the Tooth Study
There is no cost for the Tooth Study Kit, and shipping is prepaid.
To participate in the Tooth Study, download the information below
Sign up for News & Events
COPYRIGHT© 2025 IWSA / International WAGR Syndrome Association
